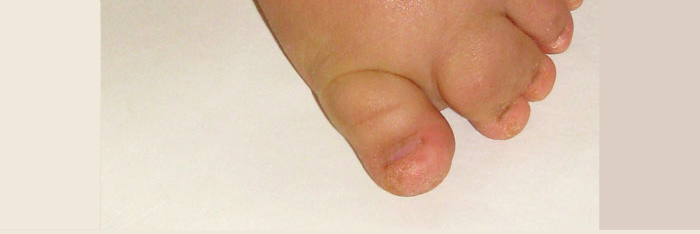
Syndrome Description
Learn more about M-CM
In June 2012, the discovery of the genetic mutation responsible for M-CM was published and the first set of clinical management guidelines was included in a supplement. The authors acknowledge that these guidelines are provisional and will surely change as understanding about M-CM grows. Note that this paper refers to M-CM as MCAP.
Links:
The guidelines summarized:
Prior to this publication, there was little consensus among researchers about the need for cancer screening in M-CM. There is no new evidence about cancer incidence in M-CM, but the gene mutation that is now thought to cause M-CM, PIK3CA, is found mutated in cancer. Additionally, PIK3CA has recently been implicated in CLOVES syndrome, where a Wilms tumor risk has been more clearly demonstrated.
Hypoglycemia
Increased reports of hypoglycemia have emerged in the scientific literature and in the patient community in association with M-CM (aka MCAP) and related megalencephaly conditions. A 2017 paper, Hypoglycaemia Represents a Clinically Significant Manifestation of PIK3CA- and CCND2- Associated Segmental Overgrowth, states the following:
"The current evaluation of children with MCAP and MPPH does not standardly include investigation of hypoglycaemia. We suggest screening for low blood sugars regularly during the neonatal period and review by an endocrinologist if hypoglycaemia is detected in patients with MCAP or MPPH to allow better preparation for illnesses or periods of fasting. Furthermore, a controlled fast may be reasonable in patients with MPPH or MCAP to identify hypoglycaemic risk prior to sedation for neuroimaging which is recommended regularly in early life."
Learn more about M-CM
Order brochures or download a PDF
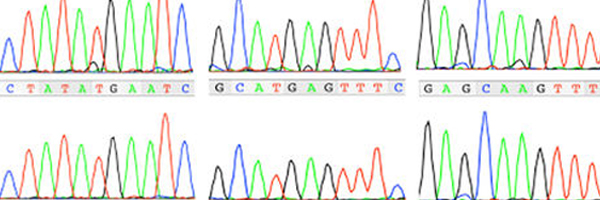
Guidance for clinical genetic testing

Explore the results of a patient survey conducted in 2012

Explore the research literature related to M-CM

An extensive list of resources